Adopting Computer Software Assurance for Validation of Computer Systems
Shortly, the FDA will be releasing a new guidance document for Computer Software Assurance (CSA) for Production and Quality System Software.
The CSA approach is broken down into 4 parts
· Intended Use
· Evaluation of Risk
· Assurance
· And Records
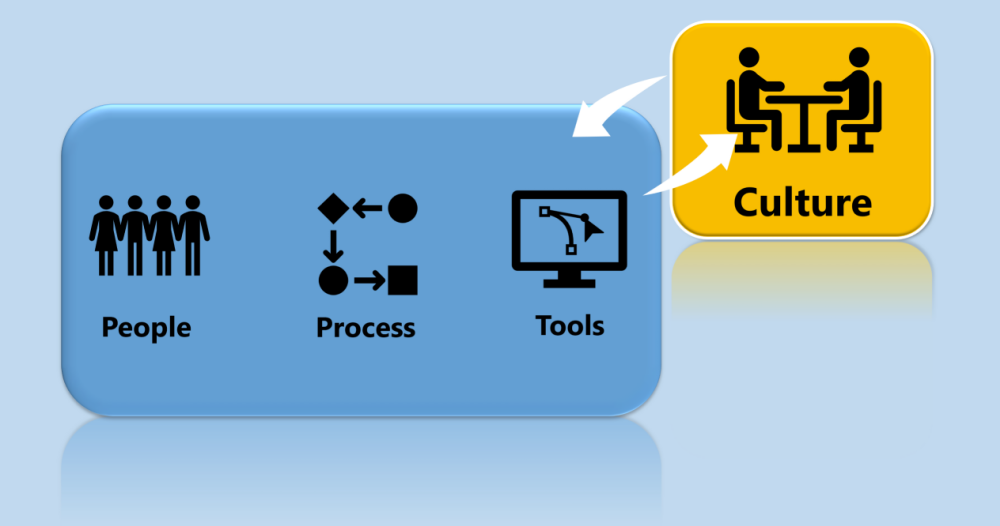
What we have found is, it’s no longer just about people, process, and tools. An organization’s culture must change to take full advantage of the soon to be released FDA guidance. Watch this video to learn more about FDA position on these 4 items plus what must be documented. Once you are ready to adopt a new approach to verification and validation, we can help. We will help you with how to properly conduct a vendor audit of a software vendor; how to streamline your validation effort to complete validation projects in days versus weeks or months; and guide your organization through the process of adopting a more Agile approach to validation.

